B. Ideal Gas Constant and Conversion Factors
Ideal Gas Constant

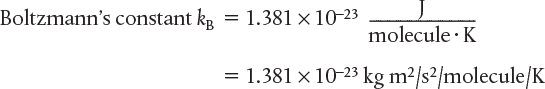
Volume of Ideal Gas
1 lb-mol of an ideal gas at 32°F and 1 atm occupies 359 ft3 (0.00279 lbmol/ft3).1 mol of an ideal gas at 0°C and 1 atm occupies 22.4 dm3 (0.0446 mol/dm3).
![]()
where CA = concentration of A, mol/dm3 P = pressure, kPa R = ideal gas constant, 8.314 kPa · dm3/mol·K T = temperature, K yA = mole fraction ...
Get Essentials of Chemical Reaction Engineering, 2nd Edition now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

