12Stress‐Migration
12.1 Introduction
Conceptually, there is a fundamental difference between stress‐migration and electromigration or thermomigration. The latter two are cross‐effects on the basis of irreversible processes, as discussed in the last three chapters. This is because the atomic flow in electromigration or thermomigration is accompanied by electron flow or heat flow, respectively. Yet, there is no “stress flow” accompanying the atomic flow in stress‐migration, especially if we assume elastic stress. Stress‐migration is a primary flow of atoms, driven by stress potential gradient, which is also a chemical potential gradient [1–3]. Often, stress‐migration is called a steady‐state diffusional creep.
Stress potential is defined as σΩ, where σ is stress and Ω is atomic volume. Thus, the driving force of stress‐migration is given as
And the atomic flux in stress‐migration is given as
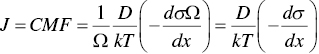
where in a pure metal, we have CΩ = 1.
A common example of steady‐state diffusional creep is the sagging of Pb pipes by their own weight under gravity in certain very old buildings. Room temperature is a relatively high temperature for Pb, whose melting point is 327 °C; therefore, atomic diffusion is sufficient for creep to occur over years. A modern application of creep is the use ...
Get Electronic Packaging Science and Technology now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

